Abstract
The imbalance of proinflammatory effector T cells (Teff) and anti-inflammatory regulatory T cells (Treg) is associated with autoimmune and inflammatory diseases. Clinical trials have demonstrated that Tregs can prevent and treat GVHD, without increasing the risk of relapse and infection (Guo et al., 2021). CD6, a costimulatory receptor is expressed on T cells including pathogenic Teff cells at high levels (CD6high), while Tregs are CD6low. Studies have demonstrated that ex-vivo depletion of CD6+ donor cells prior to hematopoietic cell transplantation (HCT) decreases the incidence of aGVHD (Soiffer et al., 1992; Soiffer et al., 1998), highlighting the importance of CD6. Notably T cells from CD6knockout mice fail to differentiate to Th17 cells but were seen to skew to a Treg phenotype more rapidly, highlighting the importance in determining Teff/Treg cell fate. Although the role of CD6 in Teff development is well characterized, here we sought to investigate the role of CD6 in the development and activity of Treg cells.
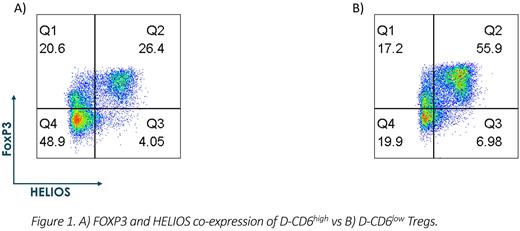
Itolizumab, a humanized anti-CD6 monoclonal antibody that modulates cell surface levels of CD6, was used to generate D-CD6low (derived CD6 low) Tregs which are Tregs derived from CD6low naïve T cells, after which itolizumab was removed and Tregs were differentiated using a standard protocol. Tregs derived from itolizumab-treated D-CD6low cells had greater co-expression of FOXP3 and HELIOS (~2-fold) vs. Tregs derived from isotype-treated CD6high (D-CD6high derived from CD6high naïve T cells)cells prior to entering a standard Treg suppression assay. These Tregs were also assessed for FOXP3 and HELIOS at the end of the suppression assay and D-CD6low Tregs cells maintained greater co-expression over those D-CD6high Tregs. Markers of active Tregs GITR and CTLA4 were also observed at higher levels on D-CD6low Tregs compared to D-CD6high Tregs. Furthermore, these D-CD6low Tregs possessed greater suppressive function, with increased inhibition of proliferation by stimulated T responder cells by at least 50% more than D-CD6high Tregs; this was verified in three different donors. Similar observations were made when measuring levels of cytokine. D-CD6low Tregs were able to suppress T responder's production of Th1 and Th17 relevant cytokines (TNFα, IL-17A, and IL-22) by 60-90% compared to D-CD6high Tregs.
This is the first study to directly characterize the role of CD6 in the development and activity of Treg cells. These data suggest that reduced levels of cell surface CD6 associated with itolizumab treatment facilitate the development of Treg cells with greater stability and suppressive activity. Consequently, modulating the levels of CD6 may improve immune tolerance during autoimmune and inflammatory diseases. Further, targeting the CD6 pathway may improve ex vivo generation of Tregs for therapies. A phase III study using itolizumab as a first line treatment in combination with steroids for patients with aGVHD is currently ongoing (NCT05263999).
Disclosures
Ampudia:Equillium: Current Employment, Current equity holder in publicly-traded company. Chu:Equillium: Current Employment, Current equity holder in publicly-traded company. Connelly:Equillium: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees. Ng:Equillium: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal